Measuring Efficacy of Periodontal Therapies in reducing Peri-Implantitis Risks: A Scientific Approach Research Protocols
PROBLEM
Peri-implantitis is a top cause of implant failure and is even more complex to treat than periodontitis
Incidence of peri-implantitis is 28.6% in patients with periodontal disease history vs 5.8% without history of gum disease.1 500,000 patients could be facing peri-implantitis 10+ years post surgery.
70% of Americans have periodontal disease doubling risk for heart disease, diabetes, pre-term birth, cancer even Alzeimers’.
Periodontal disease is the primary cause of tooth loss in patients over 40 years old.
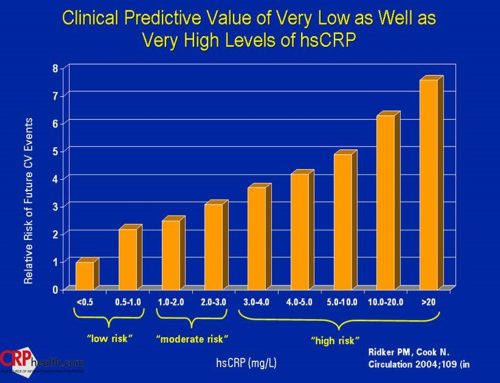
Chlorhexidine increases heart disease risks within 1 day of using twice.2
PURPOSE
Many studies show the efficacy of one therapy or homecare product but periodontitis and peri-implantitis are multi-causal.
This study measures efficacy of a combination of perio therapies used in a defined protocol and includes:
Demographics, Health history, Family Health History, Bacteria Diagnosis
Scaling and Root Planing
Clean Kiss Organic Swish Mouthwash, Scrub toothpaste, Spray breath refresher, Support Anti-Inflammatory and Bone Nutraceuticals.
Laser Assisted Periodontal Therapy
30 Second Smile Scrub Brush
Hydrofloss oral irrigator
The study will also determine how well organic botanical home care compares to control groups.
PROTOCOL Appointment 1
Document Health History, Family Health History.
Diagnostics include:
Blood Pressure
Document Full Mouth Probe Scores
If 6 or more probe scores measure >3mm, is 40+ years old offer research enrollment whether or not seeking implants or on medication
Patient signs compliance and genetic consent agreement
Complete finger stick for A1c/Crp and Salivary Oral DNA Alert 2 Gene and My Perio Path tests
Start Patient on Clean Kiss 3 month Complete Kit.
3. Show patient Clean Kiss Use video
4. Appoint Laser/SRP ASAP
Place Oral DNA order and place sample in oral DNA mailer. Schedule weekly pickup.
Document Chairside notes in Oral DNA Portal. Schedule one hour appt in ASAP.
PROTOCOL Appointment 2
Use Laser to decontaminate entire mouth
Complete 2 quadrants SRP
Calculate 1 laser tx per mm pocket depth reduction desired
Complete full mouth LAPT as first laser tx.
Re-enforce complete homecare instructions
Appoint 1 hour in 2 weeks
Document in Oral DNA Clinical Notes Portal
PROTOCOL Appointment 3
Use Laser to decontaminate entire mouth
If needed complete remaining 2 quadrants SRP
Complete second LAPT TX
Re-enforce complete homecare instructions
Appoint Implant placement surgery in 2 weeks
Document in Oral DNA Clinical Notes Portal
PROTOCOL Appointment 4
Use Laser to decontaminate entire mouth
Place implant fixture
Re-enforce homecare use to promote healing
Appoint Surgical follow up in 2 weeks
Document Patient Notes in Oral DNA Clinical Notes Portal
PROTOCOL Appointment 5
Complete Post Op healing check
3rd Laser Round, if needed*
Otherwise, complete and document Full Mouth Probe
Complete second A1c/Crp test
Complete second My Perio Path Oral DNA test
To complete healing regimen set up homecare autoship to patient or supply 3 month complete kit
Appoint Implant tooth restoration in 3-6 months
Document Patient Notes
* If 3rd laser round is needed, call back in 2 weeks to complete protocol
COMPLETE PHASE 1 RESEARCH
For patient to be included in research results oral DNA forms must be completed and entered into clinical notes portal including the following:
1. % of Bleeding on Probing is calculated by #bleeding sites/#teethx6.
2. Bone loss–Mild, Medium, Severe
3. % of Probing scores >3 is calculated by #probe scores >3mm/#teethX6.
4. % of Bleeding on Probing is calculated by #bleeding sites/#teethx6.
5. Before and after A1c and Crp Scores
6, Document if High Blood Pressure.
7. Medications= Yes or No.
8. Tooth Loss= Yes or No.
9. Oral DNA Genetic Susceptibility test
10. Before and After My Perio Path Tests
11. Document # of ounces CK Swish used
12. Document # of both supplements taken
PUBLICATION
- Participants names will be listed on white paper results
- Oral DNA Labs will market research results (and participants) nationally
- Results will be presented at the American Academy of Implant Dentist meeting
- HCP Wellnet will publish results on social media
- Participants will have access to media for their PR and Marketing
- 1 syndicated Press Release announcing your joining the study
- Team protocol training ($1,500 value)
PRODUCTS
A minimum of 10 patients are required to participate. You will need the following products to participate in the research:
Research Products for 10 patients include:
10 Alert 2 Periodontist Susceptibility and My Perio Pathogen tests (before therapy documentation)
10 My Perio Pathogen tests (documents after therapy)
20 A1c/Crp test (documents before and after therapy)
10 30 Second Smile Scrub Brush
10 Hydrofloss Oral Irrigators
10 3 month Clean Kiss Complete Kits (Each kit includes 3 32 oz bottles Swish mouthwash; 2 tubes Scrub toothpaste; 3 Spray breath refresher; 3 bottles ea Support Bone and Support Anti-Inflammatory)
No shipping
Video Player



Leave A Comment
You must be logged in to post a comment.